CLONAZEPAM Synthesis, SAR, MCQ, Structure, Chemical Properties and Therapeutic Uses
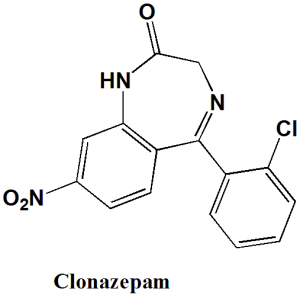
Clonazepam
IUPAC nomenclature
5-(2-Chlorophenyl)-7-nitro-1,3-dihydro-1,4-benzodiazepin-2-one
Classification
Clonazepam is a benzodiazepine derivative.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 315.71 g/mol |
| 2 | Physical appearance | Off-white to light yellow crystalline powder |
| 3 | Melting point | 237.5°C |
| 4 | Solubility | Insoluble in benzene; slightly soluble in acetone, methanol, chloroform |
| 5 | Octanol/water partition coefficient | 2.41 |
| 6 | Presence of ring | Diazepine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
Clonazepam increases the GABA affinity for the GABA receptors. Due to this, more chloride ions conducts across the neuron cell membrane which leads to the hyperpolarization of the neurons. This makes it difficult for the neurons to fire action potentials and thus there is less excitation of the neurons.
Structure Activity Relationship
- Nitrogen atoms of the amide moiety should not be substituted.
- Carbonyl carbon atom of 1,4-benzodiazepines should exhibit low electron density for proper schistosomicidal activity.
- Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
- An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
- A proton-accepting group is essential on Ring B for binding with GABAA
- When the proton accepting group is present on the 2-position of the ring B, and is in coplanar spatial orientation with Ring A, maximum activity is observed.
- Replacement of oxygen with sulfur in ring B results in alteration in the selectivity for binding with GABA BZR subpopulations, but anxiolytic properties are maintained.
- There is no effect on agonist activity, but the antagonist activity decreases when methylene 3-position or imine nitrogen of the ring B is substituted.
- Derivatives having the 3-hydroxy moiety are fast excreted.
- Sterically large substituents on ring B, like tert-butyl group reduces the receptor affinity and the in vivo activity.
- 4,5-double bond and 4-position nitrogen is not essential for anxiolystic activity.
- BZR affinity is decreased if C=N bond is replaced with C-N bond.
- 5-phenyl ring C is not necessary for the binding with BZR.
- Substitution at the para position of the ring C decreases the agonist activity of the drug.
- There is no change observed in the agonist property of the drug when there is substitution at ortho position.
- When 1,2-bond f the ring C is annelated with an additional electron rich ring such as imidazole, affinity of the BZR increases.
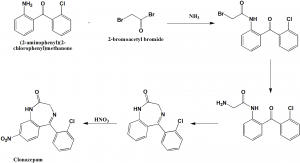
Method of synthesis
i. 2-chloro-2’nitrobenzophenone is reduced to 2-chloro-2’-aminobenzophenone by hydrogen over Raney nickel.
ii. The amino group of the above formed compound is amidated by 2-bromoacetyl bromide to produce bromoacetamide.
iii. Bromoacetamide is coverted into aminoacetamide by reaction with ammonia.
iv. On reaction with pyridine, the above formed compound cycled into 5-(2-chlorophenyl)-2,3-dihydro-1H-1,4-benzodiazepine-2-one.
v. Upon nitration, clonazepam is synthesised.
Therapeutic Uses
Clonazepam is used for:
- Prevention and control of seizures
- Treatment of panic attacks
Side Effects
Side effects of Clonazepam are:
- Dizziness
- Drowsiness
- Increased saliva production
- Loss o coordination
- Tiredness
- Depression
- Mood changes
- Suicidal thoughts
- Allergic reactions
MCQs
Q.1 “5-(2-Chlorophenyl)-7-nitro-1,3-dihydro-1,4-benzodiazepin-2-one” is the IUPAC nomenclature of which drug?
a) Aspirin
b) Clonazepam
c) Esmolol
d) Cisplatin
Q.2 Correct melting point of the drug Clonazepam is?
a) 745°C
b) 90°C
c) 106°C
d) 237.5°C
Q.3 Match the following with correct classifications of the drugs.
| i. Clonazepam | A. Vinca alkaloids cytotoxic drug |
| ii. Vincristine | B. Epipodophyllo toxin |
| iii. Etoposide | C. Benzodiazepine derivative |
| iv.Diclofenac | D. Anti-inflammatory agent |
a) i-A, ii-C, iii-D, iv-B
b) i-C, ii-A, iii-B, iv-D
c) i-D, ii-C, iii-A, iv-B
d) i-A, ii-D, iii-C, iv-B
Q.4 Mechanism of action of drug Clonazepam includes?
I. Increasing GABA affinity for GABA receptors.
II. Binding with COX-1 enzyme.
III. Prevention of conversion of arachidonic acid to thromboxane.
IV. Hyperpolarization of neuron
a) II, III, IV
b) I, IV
c) I, III, IV
d) I, II
Q.5 Correct sequence for True and False for the given statements related with the SAR of Clonazepam drugs?
- Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
- An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
a) FFTT
b) TFTF
c) TFFT
d) TTTT
Q.6 Type of ring present in the structure of clonazepam?
a) Pyrrole
b) Phenyl
c) Diazepine
d) None of the above
Q.7 The drug Clonazepam is used for?
a) Prevention of seizures
b) Control of seizures
c) Treatment of panic attacks
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-d
3-b
4-b
5-d
6-c
7-d