PRIMIDONE Synthesis, SAR, MCQ, Structure, Chemical Properties and Therapeutic Uses
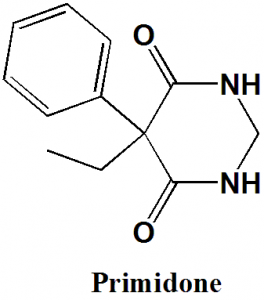
Primidone
IUPAC nomenclature
5-Ethyl-5-phenyl-1,3-diazinane-4,6-dione
Classification
Primidone belongs to barbiturate anticonvulsive drug class.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 218.25 g/mol |
| 2 | Physical appearance | White crystalline powder |
| 3 | Melting point | 281.5°C |
| 4 | Solubility | 480 mg/L in water |
| 5 | Octanol/water partition coefficient | 0.91 |
| 6 | Presence of ring | Pyrimidine, phenyl |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Phenobarbital, which is a metabolite of primidone, directly interacts with GABA-A receptors and chloride channels.
- Primidone reduces frequency of nerve firing through altering the transmembrane sodium and calcium channels transport.
Structure Activity Relationship
- Tri-keto form is most stable in aqueous solution.
- 4,6-dialcoholic tautomeric forms are least stable in aqueous solution.
- 5,5-disubstituted barbituric acid is the prime requirement for the barbituares to be sedative hypnotics.
- Esterification of either of the 1,3-diazine nitrogens decreases hypnotic activity.
- Substitution of either of the 1,3-diazine nitrogens with aliphatic carbons retains the anticonvulsive properties.
- Esterification of the 5th-position substituents yields agents with analgesic activity but with weak hypnotic properties.
- Introduction of the polar functional group at the 5th– position yields compounds which are fully devoid of sedative-hypnotic or anticonvulsive activity.
- As the number of carbons at R2 carbon increases, the lipophillicity of the drug increases.
- Modification of the 2nd-position oxygen of the barbiturate backbone with sulfur atom yields thiobarbiturate derivatives with increased lipophillicity, shorter duration of action, faster time of onset compared to oxy-derivative. [1]
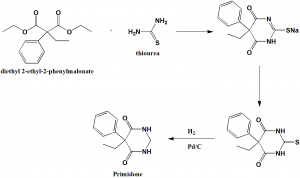
Method of synthesis
i. Diethyl phenylethylmalanoate and thiourea undergoes condensation reaction to form Phenobarbital sodium.
ii. Phenobarbital sodium acidizes to synthesize thio Phenobarbital
iii. By using lower alcohols as solvent and palladium on carbon as a catalyst, Phenobarbital undergoes high pressure hydrogenation reduction to yield primidone
Therapeutic Uses
Primidone is used for:
- Controlling seizures
Side Effects
Side effects of Primidone are:
- Dizziness
- Drowsiness
- Nausea
- Vomiting
- Headache
- Tiredness
- Excitation
- Clumsiness
- Double vision
- Decreased sexual interest
- Change in mood
- Depression
- Suicidal thoughts
- Fainting
- Slow heart beats
- Shallow breathing
- Weakness
- Pale skin
MCQs
Q.1 What can be the correct IUPAC nomenclature of Primidone?
a) 5-Ethyl-5-phenyl-1-azinane-4,6-dione
b) 5-Ethyl-5-phenyl-1,3-diazinane-4,6-dione
c) (RS)-1,3-dimethyl-3-phenyl-pyrrolidine-2,5-dione
d) (RS)-1,3-dimethyl-3-phenyl-pyrrolidine-2-one
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Primidone?
I. Tri-keto form is least stable in aqueous solution.
II. 4,6-dialcoholic tautomeric forms are least stable in aqueous solution.
III. 5,5-disubstituted barbituric acid is the prime requirement for the barbituares to be sedative hypnotics.
a) I, II
b) I
c) III
d) II, III
Q.3 Primidone can be synthesized by hydrogenation reduction of?
a) Thiophenobarbital sodium
b) Phenobarbital
c) Thiophenobarbital
d) Barbituric acid
Q.4 Side effects of drug Primidone is/are?
a) Shallow breathing
b) Decreased libido
c) Clumsiness
d) All of the above
Q.5 Match the following drugs with their correct molecular weight.
| i. Primidone | A.443.6 gm/mol |
| ii. Risperidone | B. 379.4 gm/mol |
| iii. Tiotixene | C. 410.5 gm/mol |
| iv. Droperidol | D.218.25 gm/mol |
a) i-A, ii-D, iii-C, iv-B
b) i-B, ii-A, iii-D, iv-C
c) i-A, ii-C, iii-D, iv-B
d) i-D, ii-C, iii-A, iv-B
Q.6 An example of drug from class Barbiturate anticonvulsant is?
a) Primidone
b) Esmolol
c) Methotrexate
d) Carboplatin
Q.7 The type of ring system found in the structure of Primidone is?
a) Pyridine
b) Pyrimidine
c) Cyclohexane
d) Furan
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-b
3-c
4-d
5-d
6-a
7-b