DEXTROPROPOXYPHENE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Dextropropoxyphene
IUPAC nomenclature
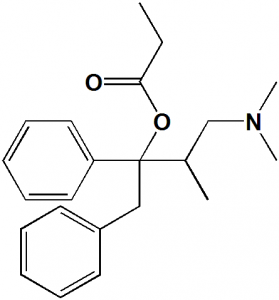
(1S,2R)-1-benzyl-3-(dimethylamino)-2-methyl-1-phenylpropyl propionate
Classification
- Dextropropoxyphene falls under category of opioid analgesics.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 339.5 g/mol |
| 2 | Physical appearance | Forms crystals from petroleum ether |
| 3 | Melting point | 75.5°C |
| 4 | Solubility | 3.32 mg / L in water |
| 5 | Octanol/water partition coefficient | 4.18 |
| 6 | Presence of ring | Phenyl |
| 7 | Number of chiral centers | 2 |
Mechanism of Action
- Dextropropoxyphene acts as a weak antagonist for OP1, OP2 andOP3 receptors within the CNS.
- It binds with G-protein receptor and produces positive and negative synaptic transmissions via G-protein which activates the effector proteins.
- Binding of drug also stimulates exchange of GTP for GDP on the G-protein complex.
- Drug also decreases the intracellular cAMP by inhibiting adenylate cyclase enzyme.
- The release of neurotransmitters like substance P, noradrenaline, acetylcholine, dopamine, GABA is inhibited by the drug.
- There is also decrease in the release of vasopressin, somatostatin, insulin and glucagon.
- Neuronal excitability is reduced by closing of the N-type voltage-operated calcium channels and opening of the calcium-dependent inwardly rectifying potassium channels, which results in hyperpolarization.
Structure Activity Relationship
SAR for Opiates can be summarized as follows:
- Replacement of phenolic hydroxyl into –OCH3/-OC2H5 will make the drug less analgesic and cough suppression will also takes place.
- Replacement of alcoholic hydroxyl with –OCH3 makes the compound 5 times more active.
- Replacement of alcoholic hydroxyl with -OC2H5 makes the compound 2.4 times more active than morphine.
- Replacement of alcoholic hydroxyl with –OCOCH3 will also activates the compound by 4.2 times.
- Replacement of alcoholic hydroxyl with ketone group inactivates the compound and makes it lesser active.
- By hydrogenation of alicyclic unsaturated linkage, activity increases by 1.2 times.
- On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity decreases.
- On replacement of N-CH3 by NCH2CH2Ph, activity increases by 14 times.
- When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Morphine antagonist.
- When the methyl group of tertiary nitrogen replaced by N(CH3)2 Cl– , compound have curare action and it do not possesses any analgesic activity.
Method of synthesis
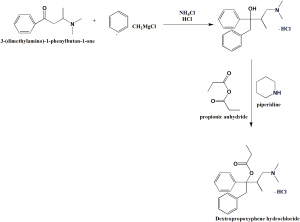
i. Addition of the secondary amine to phenypropenyl ketone to give amino ketone called beta-dimethylaminobutrophenone.
ii. Grignard reaction of the amino ketone with benzylmagnesium chloride to yield the amino, hydrochloride-carbinols 4-Dimethylamino-1,2-diphenyl-3- methyl-2-butanol Hydrochloride.
iii. Acylation of the alpha-amino carbinol hydrochloride by addition of propionic anhydride and heating to reflux.[1]
Therapeutic Uses
Dextropropoxyphene is used for:
- As an analgesic
- Relieving the symptoms of restless legs syndrome
- Easing the withdrawal symptoms
Side Effects
Side effects Dextropropoxyphene are:
- Dizziness
- Drowsiness
- Constipation
- Itching
- Fatal heart rhythm
- Nausea
- Vomiting
- Impaired alertness
- Sore throat
MCQs
Q.1 Choose the correct statements related with the physicochemical properties of drug Dextropropoxyphene.
I. Molecular weight = 339.5 gm/mol
II. It forms crystals in water
III. Melting point is 75.5oC
a) I, III
b) II
c) I
d) I, II
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Dextropropoxyphene | A. (3-carbamoyloxy-2-phenylpropyl) carbamate
|
| ii. Felbamate | B. 5-Ethyl-3-methyl-5-phenyl-imidazolidine-2,4-dione
|
| iii. Mephenytoin | C. 5H-dibenzo[b,f]azepine-5-carboxamide
|
| iv. Carbamazepine | D. (1S,2R)-1-benzyl-3-(dimethylamino)-2-methyl-1-phenylpropyl propionate
|
a) i-C, ii-A, iii-B, iv-D
b) i-D, ii-A, iii-C, iv-B
c) i-D, ii-A, iii-B, iv-C
d) i-A, ii-C, iii-B, iv-D
Q.3 Dextropropoxyphene inhibits:
I. Exchange of GTP for GDP
II. Release of noradrenaline
III. Release of acetylcholine
IV. Release of Dopmaine
a) I , II, III, IV
b)II, III, IV
c) III , IV
d) IV
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Dextropropoxyphene: Opioid analgesic
- Alosteron: Antiemetic drug
- Lysergic acid diethylamide: Hallucinogenic agent
- Tropisetron: 5-HT3 receptor antagonist
a) TFFT
b) TTTT
c) TTFF
d) FFFF
Q.5 Find the correct statement amongst the following related with the SAR of Opiates?
a) Replacement of phenolic hydroxyl into –OCH3/-OC2H5 will make the drug less analgesic
b) Replacement of alcoholic hydroxyl with –OCH3 makes the compound less active.
c) Replacement of alcoholic hydroxyl with -OC2H5 makes the compound less active
d) Replacement of alcoholic hydroxyl with –OCOCH3 will also deactivates the compound
Q.6 Number of chiral carbons present in the structure of Dextropropoxyphene is?
a) 0
b) 1
c) 2
d) 3
Q.7 Side effect of drug Dextropropoxyphene include?
a) Fatal heart rhythm
b) Itching
c) Sore throat
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-c
3-b
4-b
5-a
6-c
7-d