HALOPERIDOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Haloperidol
IUPAC nomenclature
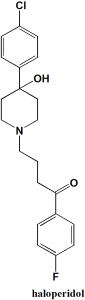
4-[4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one.
Classification
Haloperidol is a buterophenone antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 375.9 g/mol |
| 2 | Physical appearance | White to faintly yellowish in color; micro-crystalline or amorphous powder |
| 3 | Melting point | 151.5°C |
| 4 | Solubility | Freely soluble in chloroform, methanol, acetone, benzene |
| 5 | Octanol/water partition coefficient | 4.3 |
| 6 | Presence of ring | Phenyl, piperidine |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
- Haloperidol shows antipsychotic effects through strong antagonism of dopamine D2 receptor within the mesolimbic and mesocortical systems of the brain.
- The drug also has some effect on 5-HT2 and α1
- It blocks the α-adrenoceptors of the autonomic system.
Structure Activity Relationship
Structure activity relationship of butyrophenones like compounds can be described as follows:
- Electron donating group at the meta-position of the phenyl ring has highest potency.
- Potency decreases on decreasing the propyl chain of butyrophenone.
- Replacing the keto oxygen with sulfur, carbon or hydroxyl group decreases the potency.
Method of synthesis
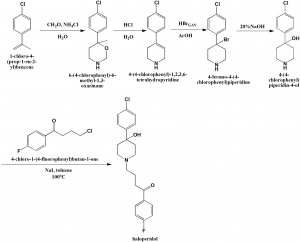
i. Ammonium chloride and formaldehyde mixture was heated to 60oC and 1-chloro-4-isopropenylbenzene is added to it to form the intermediate compound 6-(4-chlorophenyl)-6-methyl-1,3-oxazinane.
ii. 1,3-oxazine is converted into 4-(4-chlorophenyl)-1,2,3,6-tetrahydropyridine through heating.
iii. Anhydrous hydrogen bromide is passed through the above formed compound to give 4-bromo-4-phneylpiperidine derivative.
iv. The solution of the above formed derivative in water is treated with excess of 20% NaOH to produce 4-(4-chlorophenyl)piperidin-4-ol.
v. Above obtained product is heated in a closed vessel with potassium iodide in toluene and 4-chlorobutyrophenone at 100oC to get haloperidol. [1]
Therapeutic Uses
Haloperidol is used for:
- Treatment of schizophrenia
- Reducing nervousness
- Decreasing hallucinations
- Treatment of Tourette’s syndrome
Side Effects
Side effects of Haloperidol are:
- Dizziness
- Drowsiness
- Lightheadedness
- Insomnia
- Anxiety
- Muscle spasms
- Tremors
- Drooling
- Increased level of prolactin
- Decreased sexual ability in males
- Tardive dyskinesia
MCQs
Q.1 “4-[4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one” is the IUPAC nomenclature of which drug?
a) Clozapine
b) Promazine
c) Fluoxetine
d) Haloperidol
Q.2 The approximate molecular weight of the drug Haloperidol is?
a) 180.9 gm/mol
b) 375.9 gm/mol
c) 578.6 gm/mol
d) 446 gm/mol
Q.3 Match the following with correct classifications of the drugs.
| i. Haloperidol | A. Anticholinestrase |
| ii. Carbaryl | B. Nicotinic antagonist |
| iii. d-Tubocurine | C. Insecticidal Acetylcholinestrase inhibitor |
| iv. Schradan | D. Buterphenone antipsychotic drug |
a) i-B, ii-A, iii-C, iv-D
b) i-D, ii-A, iii-B, iv-C
c) i-A, ii-B, iii-D, iv-C
d) i-D, ii-B, iii-A, iv-C
Q.4 Type of interaction shown by haloperidol?
I. Antagonism Dopamine D1 receptors
II. Antagonism Dopamine D2 receptos
III. Agonism α-adrenoceptors
IV. Stimulates ß-adrenoceptor
a) I, II
b) III, IV
c) I, II, III
d) II, III, IV
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug haloperidol?
- Electron donating group at the meta-position of the phenyl ring has highest potency.
- Potency decreases on decreasing the propyl chain of butyrophenone.
- Replacing the keto oxygen with sulfur, carbon or hydroxyl group deceases the potency.
a) TFT
b) TTF
c) FFT
d) TTT
Q.6 Number of chiral carbons present in the structure of haloperidol?
a) 0
b) 1
c) 2
d) 3
Q.7 The drug Haloperidol is mainly used for?
a) Reducing nervousness
b) Treatment of Schizophrenia
c) Reducing hallucinations
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-b
3-b
4-a
5-d
6-b
7-d