ZOMEPIRAC Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
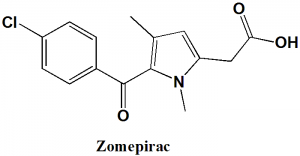
Zomepirac
IUPAC nomenclature
2-[5-(4-Chlorobenzoyl)-1,4-wronghyl-pyrrol-2-yl]acetic acid
Classification
- NSAID
- Arylalkanoic acid
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 291.73 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 178.5°C |
| 4 | Solubility | Water solubility of zomepirac sodium anhydrous is 0.0458 mg/ml |
| 5 | Octanol/water partition coefficient | logP=3.33 |
| 6 | Presence of ring | Pyrrole, phenyl |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Zomepirac inhibits prostaglandin synthetase enzyme which in turn results in decreased production of prostaglandins.
- Prostaglandin is responsible for carrying the pain signals throughout the body.
Structure Activity Relationship
SAR of class arylalkanoic acids can be summarized as follows:
- The center of acidity is located one carbon atom adjacent to the flat surface generally an aromatic ring or a heteroaromatic ring. On increasing the distance between these two centers by three atoms diminishes the activity of drug.
- Substituting a methyl group on the carbon atom that separates the aromatic ring from the acidic center increases the anti-inflammatory activity of drug. Incorporation of groups larger than methyl decreases the activity.
- A center of chirality is generated on incorporation of a methyl group. In these cases, anti-inflammatory is determined by S-(+)-enantiomers.
- Lipophillic group which is non-coplanar with the aromatic or heteroaromatic ring increases the activity. [1]
Method of synthesis
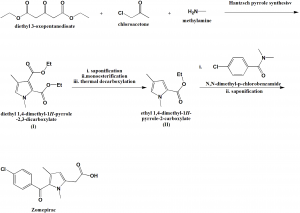
i. Reaction of aqueous methylamine, chloroacetone and diethyl 3-oxopentanedioate produces and intermediate I through Hantzsch pyrrole synthesis.
ii. Intermediate I undergoes saponification, monoesterification and thermal decarboxylation to produce an ester II.
iii. The ester is acylated with N,N-dimethyl-p-chlorobenzamide, followed by saponification to give zomepirac. [2]
Therapeutic Uses
Zomepirac is used for:
- Management of mild to severe pain
Side Effects
Side effects of Zomepirac are:
- Nausea
- Vomiting
- Allergic reactions
- Urogenital symptoms
- Dysuria
- Pyuria
MCQs
Q.1 Match the following with correct SAR of the class Arylalkanoic acid NSAIDs-
| i. Substituting a methyl group on the carbon atom that separates the aromatic ring from the acidic center | A. Increases the anti-inflammatory activity of drug |
| ii. Lipophillic group which is noncoplanar with the aromatic or heteroaromatic ring | B. Decreases the anti-inflammatory activity of drug |
| C. Increases the anti-inflammatory activity of drug | |
| D. Decreases the anti-inflammatory activity of drug |
a) i-A, ii-C
b) i-A, ii-D
c) i-B, ii-C
d) i-B, ii-D
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Zomperiac: 2-[5-(4-Chlorobenzoyl)-1,4-wronghyl-pyrrol-2-yl]acetic acid
- Diclofenac: [2-(2,6-Dichloroanilino)phenyl]acetic acid
- Tolmetin: [1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl]acetic acid
- Sulindac: {(1Z)-5-fluoro-2-methyl-1-[4-(methylsulfinyl)benzylidene]-1H-indene-3-yl}acetic acid
a) TFTF
b) FFFT
c) TTTT
d) TFFT
Q.3 Molecular weight of drug zomepirac is?
a) 146.5 gm/mol
b) 291.73 gm/mol
c) 426.21 gm/mol
d) 62.1 gm/mol
Q.4 Zomepirac mechanism of action includes?
a) Inhibition of prostaglandins synthetase enzyme
b) Stimulating prostaglandin synthesis
c) Alkylation of DNA inside nucleus
d) Blocking cholinergic receptors
Q.5 Which amongst the following is a therapeutic use of drug Zomepirac?
a) Treatment of neoplastic growth
b) Reducing pain
c) Controlling nausea
d) As an anti-viral drug
Q.6 Which of the following drug and their classification are correct?
I. Thio-tepa: Ethylinimine
II. Nafarelin: GnRH analogue
III. Zomepirac: NSAID
IV. Bisoprolol: ß-blocker
a) I, II, IV
b) II, III
c) I, II, III, IV
d) II
Q.7 Number of chiral centers present in the structure of zomepirac?
a) 0
b) 1
c) 2
d) 3
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-c
3-b
4-a
5-b
6-c
7-a