Matter, Properties of matter : Gases, Aerosol – inhalers and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Gases are compressible fluid and has no definite shape.
Properties of Gases:
1) A sample of gas assumes both the shape and volume of the container.
2) Gases are compressible.
3) The densities of gases are much smaller than those of liquids and solids and are highly variable depending on temperature and pressure. 4) Gases form homogeneous mixtures (solutions) with one another in any proportion.
The Gas Laws: 1) Boyle ‘s law : This law states that the pressure of a fixed amount of gas at a constant temperature is inversely proportional to the volume of the gas.
P α 1/V Pressure is inversely proportional to the volume
2) Charles Law: This law states that the pressure of a fixed amount of gas at a constant At a fixed pressure, the volume of a gas is proportional to the temperature of the gas.
V α T
3) Gay-Lussac’s law: This law is a special case of ideal gas law. This law applies to ideal gases held at a constant volume allowing only the pressure and temperature to change.
P1/T1 = P2/T2
4) Avogadro law: This law states that the volume of a sample gas is directly proportional the number of moles in the sample at constant temperature and Pressure.
V α n or V1/n1 = V2/n2
Kinetic Molecular Theory of Gases: The basic assumptions of the Kinetic Molecular Theory are
- The volume occupied by the individual particles of a gas is negligible compared to the volume of the gas itself.
- The particles of an ideal gas exert no attractive forces on each other or on their surroundings.
- Gas particles are in a constant state of random motion and move in straight lines until they collide with another body.
- The collisions exhibited by gas particles are completely elastic; when two molecules collide, total kinetic energy is conserved.
- The average kinetic energy of gas molecules is directly proportional to absolute temperature only; this implies that all molecular motion ceases if the temperature is reduced to absolute zero.
Liquefaction of Gases: When pressure on a gas is increased, its molecules closer together, and its temperature is reduced, which removes enough energy to make it change from the gaseous to the liquid state.
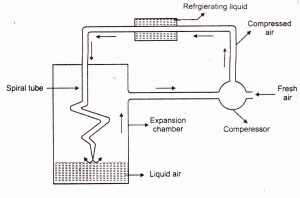
Fig 1 – Method of liquefaction of gases (taken from Chemistry skills)
Aerosols-inhaler: An aerosol is a suspension of fine solid particles or liquid droplets, in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog, dust, forest exudates and geyser steam. Examples of anthropogenic aerosols are haze, particulate air pollutants and smoke.
Liquefaction of a gas can be achieved by applying pressure on it and keep the temperature below the critical temperature. When the pressure is reduced, the molecules expand and the liquid reverts back to the gaseous state. Aerosols are based on the principle of reversible change of state on the application and release of pressure. In pharmaceutical aerosols, a drug is dissolved or suspended in a propellant, a material which exists as a liquid under the pressure conditions prevalent inside the container but gets converted to a gas under normal atmospheric conditions. The container is designed in such a manner that on depressing a valve, some of the drug propellant mixture is expelled out due to the excess pressure inside the container. The propellant used in such a products are generally fluorinated hydrocarbons although gases such as nitrogen and carbon dioxide and also being used.
The aerosol containers are filled either by cooling the propellant and drug to a low temperature within the container which is then sealed with the valve. Alternatively, the drug is sealed in the container at room temperature and the required quantity of propellant is forced into the container under pressure. In both the cases, when the container is at room temperature, part of the propellant is in the gaseous state and exerts pressure necessary to extrude the drug while the remaining is in the liquid state and provides a solution or suspension vehicle for the drug.
Inhalers: An inhaler is a device holding a medicine that you take by breathing in (inhaling). Inhalers are often used to treat chronic obstructive pulmonary disease (COPD). They are three types: Metered-dose inhaler, Dry powder inhalers, Nebulizers.
There are three types of Inhalers:
Metered -dose inhalers – The most common type of inhaler is the pressurized metered-dose inhaler (MDI) which is made up of 3 standard components- a metal canister, plastic actuator, and a metering valve.
Dry powder inhalers – Dry powder inhalers (DPI) release a metered or device-measured dose of powdered medication that is inhaled through a DPI device.
Nebulizers – Nebulizers supply the medication as an aerosol created from an aqueous formulation.
Multiple choice questions (MCQs)
1.Gases are compressible fluid and has no definite shape.
a)true
b)false
2.If a gas expands at constant temperature, it indicates that:
a)Number of the molecules of the gas increases
b)Kinetic energy of molecules decreases
c)Pressure of the gas increases
d)Kinetic energy of molecules remains the same
3.In order to increase the volume of a gas by 10%, the pressure of the gas should be
a)Increased by 10%
b)Increased by 1%
c)Decreased by 10%
d)Decreased by 1%
4.In the equation of state of an ideal gas PV = n RT, the value of the universal gas constant would depend only on
a)The nature of the gas
b)The pressure of the gas
c)The units of the measurement
d)None of the above
5.Which one of the following statements is NOT true about the effect of an increase in temperature on the distribution of molecular speeds in a gas?
a)The most probable speed increases
b)The fraction of the molecules with the most probable speed increases
c)The distribution becomes broader
d)The area under the distribution curve remains the same as under the lower temperature
6.The greater the speed of gas particles in a container, the
a)Fewer collisions there will be
b)Lower the temperature
c)Greater the pressure
d)Lower the pressure
7.According to Boyle’s law, when the pressure of a gas increases at constant temperature, its volume
a)Increases
b)Stays constant
c)Decreases
d)Increases, then decreases
8.When the temperature of a gas decreases at constant volume, its
a)Pressure increases
b)Mass increases
c)Pressure decreases
Particles move more slowly
9.According to Charles’s law, when the temperature of a gas increases at constant pressure, its
a)Volume increases
b)Mass increases
c)Volume decreases
d)Particles move very slowly
10.Boyle’s law states that for a fixed amount of gas at a constant
a)pressure, the volume increases as the temperature decreases.
b)temperature, the volume increases as the pressure increases.
c)temperature, the volume decreases as the pressure increases.
d)pressure, the volume decreases as the temperature increases.
11.A gas will approach ideal behaviour at
a)High temperature, low pressure
b)Low temperature, high pressure
c)Low temperature, low pressure
d)High temperature, high pressure
12.Which of the following are examples of natural aerosols?
a)fog
b)dust
c)forest exudates
d)all of the above
13.Dalton’s law of partial pressures is applicable to which one of the following systems?
a)CO+H2
b)H2+Cl2
c)NO+O2
d)NH3+HCl
14.The propellant used in aerosol products are generally
a)fluorinated hydrocarbons
b)nitrogen
c)carbon dioxide
d)all of the above
15. Which of the following are types of inhalers?
a)Metered-dose inhaler
b)Dry powder inhalers
c)Nebulizers
d)all of the above
Solutions:
- a)true
- d) kinetic energy of molecules remains the same
- c) decreased by 10%
- c) the units of the measurement
- b) The fraction of the molecules with the most probable speed increases
- c) greater the pressure
- c) decreases
- c) pressure decreases
- a) volume increase
- c) temperature, the volume decreases as the pressure increases
- a) high temperature, low pressure
- d)all of the above
- a) CO+H2
- d)all of the above
- d)all of the above
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 1-16.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 49-56.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test