DIPHENHYYDRAMINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
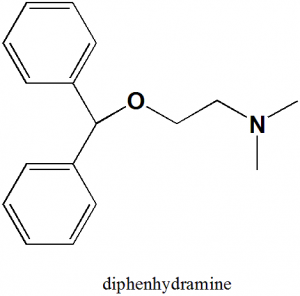
Diphenhydramine
IUPAC nomenclature
2-(diphenylmethoxy)-N,N-dimethylethanamine
Classification
- H1-receptor antihistamine
- Diphenylmethane
- Ethanolamine ether antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 255.35 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 168oC |
| 4 | Solubility | 3060 mg/L |
| 5 | Octanol/water partition coefficient | 3.27 |
| 6 | Presence of ring | Phenyl ring |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- It is an inverse agonist for H1 receptors which reverses the effects of histamine on capillaries and thus, reduces the symptoms of allergic reactions.
- It crosses the blood brain barrier and inversely agonizes H1 CNS receptors to induce drowsiness, and suppresses the medullary cough center.
- It competitively antagonizes the muscarinic acetylcholine receptors. Therefore, it can be used as an antiparkinson medication.
- It also shows local anesthetic properties by showing activity as an intracellular sodium channel blocker.
Structure Activity Relationship
Structure activity of ethanolamine ethers antihistamines can be summarized as:
- 8-chlorotheophyllinate salt of diphenhydramine is used for treatment of motion sickness
- Compounds with p-Cl-Ph and 2-pyridyl aryl groups are carboxamine, which is a potent anti-histamine drug.
- Substitution of the methyl group at the carbon alpha to the ether function gives a related compound known as doxylamine.
- An additional carbon atom between oxygen and nitrogen produces clemastine which has lower sedative properties.
- In setastine, alkyl amine substituent is incorporated into seven-membered hexahydroazepine ring, having lower sedative properties.
- With increase in alkyl group size at C-2’, there is decrease in antihistaminic activity and increases in anticholinergic activity..
- Introduction of alkyl substituents at C-4’results in decrease in anticholinergic activity and increase in antihistaminic activity.[1]
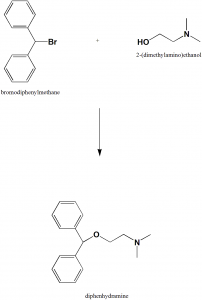
Method of synthesis
- Reaction of benzhydrylbromide and 2-dimethylaminoethanol produces diphenhydramine. [2]
Medicinal Uses
Diphenhydramine is used for treatment of:
- Sneezing
- Runny nose
- Watery eyes
- Allergy symptoms
- Watery eyes
- Itching
- Motion sickness
- Inducing sleep
- Parkinson’s disease
Side Effects
Side effects of diphenhydramine are:
- Dizziness
- Drowsiness
- Dry mouth and nose
- Loss of coordination
- Upset stomach’
- Constipation
- Blurred vision
- Dry eyes
- Pounding heartbeats
- Difficulty in urination
- Confusion
- Uncontrollable movement of tongue.
MCQs
Q.1 “2-(diphenylmethoxy)-N,N-dimethylethanamine” is the IUPAC nomenclature of which drug?
a) Diphenhydramine
b) Chlorpheniramine
c) Terbutaline
d) Cetrizine
Q.2 Molecular weight of Diphenhydramine is?
a) 300.23 gm/mol
b) 255.35 gm/mo
c) 190.0 gm/mol
d) 134.32 gm/mol
Q.3 Match the following with correct classifications of the drugs.
| i. Diphenhydramine | A. Ethanolamine ether antihistamine drug |
| ii. Dactinomycin | B. H2-antagonist |
| iii. Clofibrate | C. Anti-hyperlipidemic agent |
| iv. Cimetidine | D. Anti-biotics anti-neoplastic drug |
a. i-A, ii-C, iii-B, iv-D
b. i-C, ii-D, iii-A, iv-B
c. i-D, ii-,B iii-A, iv-C
d. i-A, ii-D, iii-C, iv-B
Q.4 Which of the following mechanisms are involved for the action of diphenhydramine drug?
I. Inverse agonist for H1 receptors
II. Inverse agonist for H2 receptors
III. Activity as an intracellular sodium channel blocker
IV. Competitively antagonizing the action of muscarinic acetylcholine receptors.
a) I, IV
b) II, III, IV
c) I, III, IV
d) I, II, III
Q.5 Correct sequence for True and False for the given statements related with the SAR of class ethanolamine ethers antihistamines can be?
- 8-chlorotheophyllinate salt of diphenhydramine is used for treatment of motion sickness
- Compounds with p-Cl-Ph and 2-pyridyl aryl groups are carboxamine, which is a potent anti-histamine drug.
- Substitution of the methyl group at the carbon alpha to he ether function gives a related compound known as doxylamine.
- An additional carbon atom between oxygen and nitrogen produces clemastine which has lower sedative properties.
a) TTFF
b) TFTF
c) TTTT
d) FTFF
Q.6 Diphenhydramine can be synthesized by the reaction of benzhydrylbromide with?
a) 2-dimethylaminoethanol
b) p-aminobenzoic acid
c) Benzoic acid
d) Butanoic acid
Q.7 The drug diphenhydramine is mainly used for?
a) Treatment of sneezing
b) Treatment of watery eyes
c) Treatment of motion sickness
d) All of the above
Successful GPATINDIAN Candidates
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-b
3-d
4-c
5-c
6-a
7-d
REFERENCES
[1] Lemke TL, Williams DA, editors. Foye’s principles of medicinal chemistry. Lippincott Williams & Wilkins; 2012 Jan 24. [2] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.